Imagine taking all the mass in the Milky Way (estimated to be around a trillion solar masses) and collapsing it into a black hole. The result wouldn’t be an ordinary black hole. Not even to astrophysicists, for whom all sorts of weird shit is ordinary.
The largest black hole candidate is the black hole at the center of the quasar S5 0014+813, estimated at 40 billion solar masses. In other words, almost a hundred times smaller than our hypothetical hole. As I said last time, as far as astronomical objects go, black holes are a fairly comfortable size. Even the largest don’t get much bigger than a really large star. Here, though, is how big our trillion-sun black hole would be, if we replaced the sun with it:

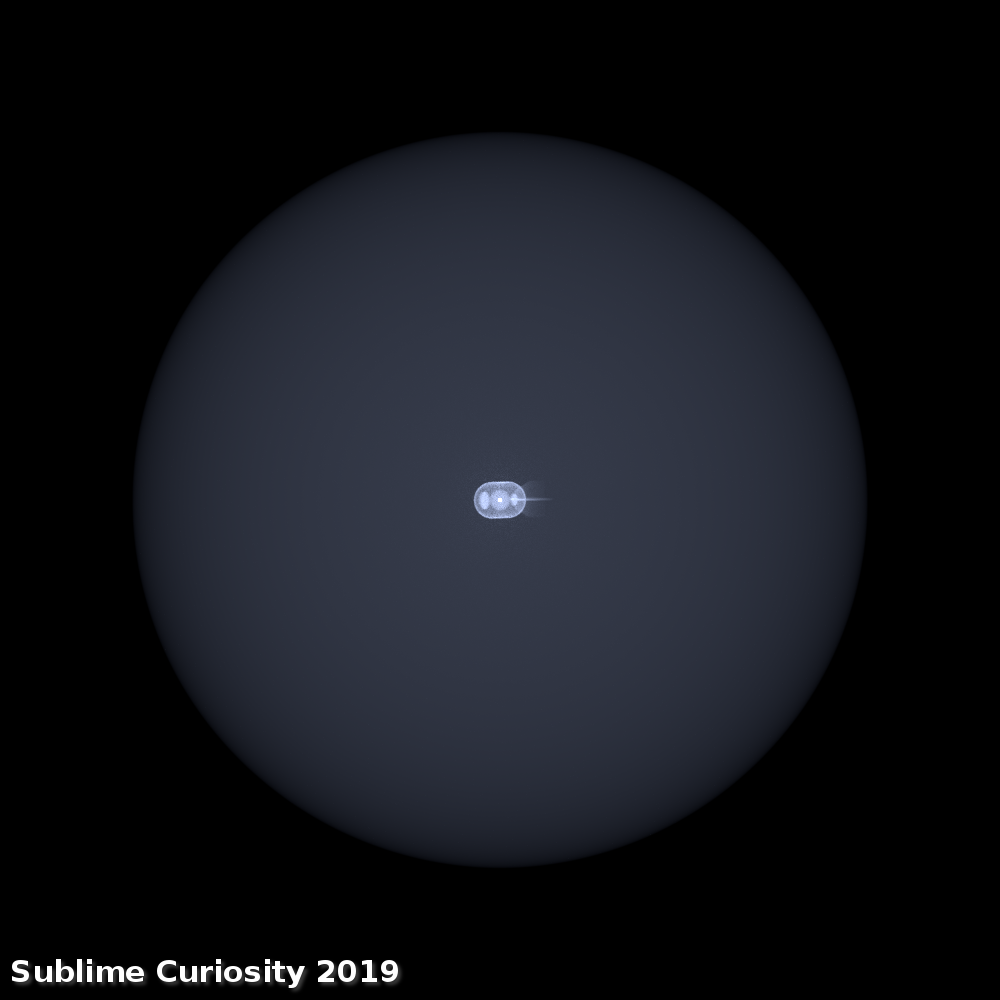
(Rendered in Universe Sandbox 2.)
The thing circled in orange is the black hole. When I started tinkering with the simulation, I was kinda hoping there’d be one or two dwarf planets outside the event horizon, so their orbits could at least offer a sense of scale. No such luck: the hole has a Schwarzschild radius of 0.312 light-years, which reaches well into the Oort cloud. That is, the galaxy-mass black hole’s event horizon alone would extend beyond the heliopause, and would therefore reach right into interstellar space. Proxima Centauri, around 4.2 light-years from Earth, is circled in white.
The immediate neighborhood around a black hole like this would be rough. We’re talking “feral children eating the corpse of a murder victim while two garbagemen fight to the death with hatchets over who gets to empty the cans on this street” kind of rough. That kinda neighborhood. No object closer than half a light-year would actually be able to orbit the hole: it would either have to fall into the hole or fly off to infinity.
That is, of course, if the hole isn’t spinning. As I said last time, you can orbit closer to a spinning hole. But I’m going to make a leap here and say that our galaxy-mass black hole isn’t likely to be spinning very fast. Some rough calculations suggest that, if it were rotating at half the maximum speed,the rotational kinetic energy alone would have several billion times the mass of the sun. I’m going to assume there’s not enough angular momentum in the galaxy to spin a hole up that much. I could be wrong. Let me know in the comments.
Spin or no spin, it’s gonna be a rough ride anywhere near the hole. Atoms orbiting at the innermost stable orbit (the photon sphere) are moving very close to the speed of light, and therefore, to them, the ambient starlight and cosmic microwave background ahead of them is blue-shifted and aberrated into a horrifying violet death-laser, while the universe behind is red-shifted into an icy-cold nothingness.
But, as we saw last time, once you get outside a large hole’s accretion disk, things settle down a lot. When it comes to gravity and tides, ultra-massive black holes like these are gentle giants. You could hover just outside the event horizon by accelerating upwards at 1.5 gees, which a healthy human could probably tolerate indefinitely, and which is very much achievable with ordinary rocket engines. The tides are no problem, even right up against the horizon. They’re measured in quadrillionths of a meter per second per meter.
Of course, if you’re hovering that close to a trillion-solar-mass black hole, you’re still going to die horribly. Let’s say your fuel depot is orbiting a light-year from the hole’s center, and they’re dropping you rocket fuel in the form of frozen blocks of hydrogen and oxygen. By the time they reach you, those blocks are traveling at a large fraction of the speed of light, and will therefore turn into horrifying thermonuclear bombs if you try to catch them.
But, assuming its accretion disk isn’t too big and angry, a hole this size could support a pretty pleasant galaxy. The supermassive black hole suspected to lie at the center of the Milky Way makes up at about 4.3 parts per million of the Milky Way’s mass. If the ratio were the same for our ultra-massive hole, then it could host around 200 quadrillion solar masses’ worth of stars, or, in more fun units, 80,000 Milky Ways. Actually, it might not be a galaxy at all: it might be a very tightly-packed supercluster of galaxies, all orbiting a gigantic black hole. A pretty little microcosm of the universe at large. Kinda. All enclosed within something like one or two million light-years. A weird region of space where intergalactic travel might be feasible with fairly ordinary antimatter rockets.
You’ll notice that I’ve skipped an important question: Are there any trillion-solar-mass black holes in the universe? Well, none that we know of. But unlike some of the other experiments to come in this article, black holes this size aren’t outside the realm of possibility.
I frequently reference a morbid little cosmology paper titled A Dying Universe. If you’re as warped as I am, you’ll probably enjoy it. It’s a good read, extrapolating, based on current physics, what the universe will be like up to 10^100 years in the future (which they call cosmological decade 100). If you couldn’t guess by the title, the news isn’t good. A hundred trillion years from now (Cosmological Decade 14), so much of the star-forming stuff in galaxies will either be trapped as stellar corpses or will have evaporated into intergalactic space that new stars will stop forming. The galaxies will go dark, and the only stars that shine will be those formed by collisions between high-mass brown dwarfs. By CD 30 (a million trillion trillion years from now), gravitational encounters between stars in the galaxy will have given all the stars either enough of a forward kick to escape altogether, or enough of a backward kick that they fall into a tight orbit around the central black hole. Eventually, gravitational radiation will draw them inexorably into the black hole. By CD 30, the local supercluster of galaxies will consist of a few hundred thousand black holes of around ten billion solar masses, along with a bunch of escaping rogue stars. By this time, the only source of light will be very occasional supernovae resulting from the collisions of things like neutron stars and white dwarfs. Eventually, the local supercluster will probably do what the galaxy did: the lower-mass black holes will get kicked out by the slingshot effect, and the higher-mass ones will coalesce into a super-hole that might grow as large as a few trillion solar masses. Shame that everything in the universe is pretty much dead, so no cool super-galaxies can form. But the long and the short of it is that such a hole isn’t outside the realm of possibility, although you and I will never see one.
The Opposite Extreme
But what about really tiny black holes? In the first post in this series, I talked about falling into a black hole with the mass of the Moon. But what about even smaller holes?
Hobo Sullivan is a Little Black Pinhole
Yeah, I feel like that sometimes. I mass about 131 kilograms (unfortunately; I’m working on that). If, by some bizarre accident (I’m guessing the intervention of one of those smart-ass genies who twist your wishes around and ruin your shit), I was turned into a black hole, I’d be a pinprick in space far, far smaller than a proton. And then, within a tenth of a nanosecond, I would evaporate by Hawking radiation (if it exists; we’re still not 100% sure). When a black hole is this small, Hawking radiation is nasty shit. It would have a temperature of a hundred million trillion degrees, and I’d go off like four Tsar Bombas, releasing over 200 megatons of high-energy radiation. Not enough to destroy the Earth, but enough to ruin the year for the inhabitants of a medium-sized country.
There’s no point in trying to work out things like surface tides or surface gravity: I’d be gone so fast that, in the time between my becoming a black hole and my evaporation, a beam of light would have traveled a foot or two. Everything around me is as good as stationary for my brief lifetime.
A Burial Fit for a Pharaoh. Well, for a weird pharaoh.
Things change dramatically once black holes get a little bigger. A hole with the mass of the Great Pyramid of Giza (around 6 billion kilograms) would take half a million years to evaporate. It would still be screaming-hot: we’re talking trillions of Kelvin, which is hot enough that nearby matter will vaporize, turn to plasma, the protons and neutrons will evaporate out of nuclei, and then the protons and neutrons themselves will melt into a quark-gluon soup. But, assuming the black hole is held in place exactly where the pyramid once stood, we won’t see that. We’ll only see a ball of plasma and incandescent air the size of a university campus or a big football stadium, throbbing and booming and setting fire to everything for a hundred kilometers in every direction. The Hawking radiation wouldn’t inject quite enough energy to boil the planet, but it would probably be enough (combined with things like the fact that it’s setting most of Egypt on fire) to spoil the climate in the long run.
This isn’t an issue if the black hole is where black holes belong: the vacuum of space. Out there, the hole won’t gobble up Earth matter and keep growing until it destroys us. Instead, it’ll keep radiating brighter and brighter until it dies in a fantastic explosion, much like the me-mass black hole did.
Can’t you just buy a space heater like a normal person?
It’s starting to get cold here in North Carolina. Much as I love the cold, I’ve been forced to turn my heater on. But, you know, electric heating is kinda inefficient, and this house isn’t all that well insulated. I wonder if I could heat the house using Hawking radiation instead…
Technically, yes. Technically in the sense of “Yeah, technically the equations say yes.” Technically in the same way that you could technically eat 98,000 bacon double cheeseburgers at birth and then go on a 75-year fast, because technically, that averages out to 2,000 Calories per day. What I mean is that while the numbers say you can, isolated equations never take into account all the other factors that make this a really terrible idea.
A black hole with the mass of a very large asteroiod (like Ceres, Vesta, or Pallas) would produce Hawking radiation at a temperature of 500 Kelvin, which is probably too hot to cook with, but cool enough not to glow red-hot. That seems like a sensible heat source. Except for the fact that, as soon as you let it go, it’s going to fall through the floor, gobble up everything within a building-sized channel, and convert that everything into superheated plasma by frictional effects as it falls into the hole. And except for the fact that if you’re in the same neighborhood as the hole, you’ll simultaneously be pulled into it at great speed by its gravity, and pulled apart into a bloody mass of fettuccine by tidal forces. And except for the fact that, as the black hole orbits inside the Earth, it’s going to open up a kilometer-wide tunnel around it and superheat the rock, which will cause all sorts of cataclysmic seismic activity, and ultimately, the Earth will either collapse into the hole, or be blasted apart by the luminosity of the forming accretion disk, or some combination thereof.
Back to the Original Extreme
But there’s one more frontier we haven’t explored. (I was watching Star Trek yesterday.) That is: the biggest black hole we can reasonably (well, semi-reasonably) imagine existing. That’s a black hole with a mass of around 1 x 10^52 kilograms: a black hole with the mass of the observable universe. Minus the mass of the Earth and the Sun, which make less of a dent in that number than stealing a penny makes a dent in Warren Buffett’s bank account.
The hole has a Schwarzschild radius of about 1.6 billion light-years, which is a good fraction of the radius of the observable universe. Not that the observable universe matters much anymore: all the stuff that was out there is stuck in a black hole now.
For the Earth and Sun, though, things don’t change very much (assuming you set them at a modest distance from the hole). After all, even light needs over 10 billion years to circumnavigate a hole this size. Sure, the Earth and Sun will be orbiting the hole, rather than the former orbiting the latter, but since we’re dealing with gravitational accelerations less than 3 nanometers per second per second, and tides you probably couldn’t physically measure (4e-34 m/s/m at the horizon, and less further out, which falls into the realm of the Planck scale), life on Earth would probably proceed more or less as normal. The hole can’t inflict any accretion-disk horror on the Sun and Earth: there’s nothing left to accrete. Here on Earth, we’d just be floating for all eternity, living our lives, but with a very black night sky. If we ever bothered to invent radio astronomy, we’d probably realize there was a gigantic something in the sky, since plasma from the Sun would escape and fall into a stream orbiting around the hole, but we’d never see it. What a weird world that would be…
Then again, if the world’s not weird by the end of one of my articles, then I’m really not doing my job…